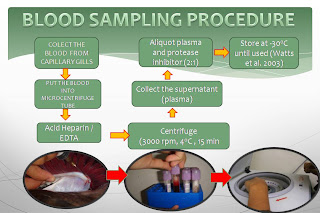
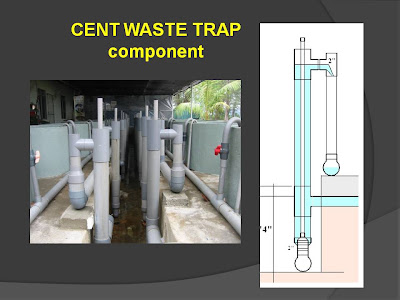
CENTS
Ahad, 18 Ogos 2013
Rabu, 21 Oktober 2009
Ahad, 28 Jun 2009
Biological Filtration and the Nitrification Cycle

A biological filter is quite simply the heart of the RAS system. It's purpose is to convert the waste matter produced by the fish from harmful ammonia into less toxic waste. It is less important to remove solids particles from water than it is to process nitrogen, so if there is to be a compromise between mechanical and biological, err on the side of biological.In other words, it is much better to allow particles below a certain size to escape back into the pond,while converting a great deal of ammonia to nitrate, than it is to catch every little thing down to a micron or less which in the process would slow the water down to the point where the bacteria have a hard time living (because they're not getting enough oxygen). The bacteria that convert ammonia to nitrate for us are among a class of bacteria that you may have heard of before. They are the so-called, “nitrogen fixing” bacteria.
This means that they take nitrogen that is unavailable to plants in its ammoniacal form, and make it available to plants in an oxidized form.There are 2 types of bacterial species that colonise the biological filter media. Nitrosomonas sp.bacteria which oxidize ammonia to nitrite, and Nitrobacter bacteria convert nitrite to nitrate.
NH3 + CO2 + 1.5 O2 + Nitrosomonas → NO2- + H2O + H+NO2- + CO2 + 0.5 O2 + Nitrobacter → NO3-
The conversion of ammonia to nitrates is performed primarily by bacteria and other nitrifying bacteria. The primary stage of nitrification, the oxidation of ammonia (NH3) is performed by bacteria such as the Nitrosomonas species, which converts ammonia to nitrites (NO2-). Other bacterial species, such as the Nitrobacter, are responsible for the oxidation of the nitrites into nitrates (NO3-).It is important for the nitrites to be converted to nitrates because accumulated nitrites are toxic to plant lifeDenitrificationDenitrification is the reduction of nitrites back into the largely inert nitrogen gas (N2), completing the nitrogen cycle.
This process is performed by bacterial species such as Pseudomonas and Clostridium in anaerobic conditions.[1] They use the nitrate as an electron acceptor in the place of oxygen during respiration. These facultatively anaerobic bacteria can also live in aerobic conditions.AmmoniaAmmonia (NH3) is produced by fish as part of their normal metabolic function and is excreted from the gills. The amount of ammonia produced is directly related to the amount of food they eat. Approximately 3-4% of normal 30-40% protein level food will be excreted as ammonia, i.e. for every 100grams of food 3-4grams (3000-4000mg) of ammonia is produced. Fish exposed to unacceptable levels of ammonia risk damage to gills, eyes, fins and skin which can result in them being susceptible to secondary bacterial infection. Using standard drop type tests kits any ammonia reading is considered unacceptable and remedial action should be taken.
NitriteAmmonia is oxidized by the Nitrosomonas sp. bacteria in the filter to produce nitrite (NO2). Whilst it is not considered as dangerous as ammonia it can still do serious damage to your fish. High levels of nitrite are likely to stress your fish leaving them susceptible to secondary infection. As with ammonia, target levelsshould be that nitrite is undetectable. Before the fish pond filter can efficiently remove ammonia and nitrite from the fish pond water, it must first become fully colonized with nitrifying bacteria. This can take some time and is a process known as fish pond filter "maturation". Each time a fish is put in the fish pond it will add to the total amount of ammonia being produced. The ammonia level in the fish pond will therefore increase slightly. Because there is more ammonia for the bacteria to utilize, they start to multiply until there are enough to use all of theammonia being produced inside the fish pond. The ammonia level in your fish pond will then fall back to zero.
Nitrate
As the ammonia level falls, the amount of nitrite produced by the bacteria in the fish pond filter will start to increase. Therefore, the level of nitrite in the fish pond will rise. The increasing nitrite level means that the bacteria that break it down can start to multiply in the fish pond filter until, as with the ammonia, there are enough to use up all the nitrite that is being produced. The nitrite level within the fish pond can then fall to zero. As this occurs, the nitrate level increases. Conversion of nitrite to nitrate (NO3) is the final stage of the nitrification process. There is debate as to the possible problems that elevated levels of nitrate may cause.Nitrate and it causes no problem at all. High nitrate may also attribute to green water(phytoplankton). The green water problem can get worst when you clean the biofilter and make water change outs, due to the reduction in bacteria.The bacteria also produces a certain phytoplankton-killing enzyme. As algae starts to grow in the biofilter,or on the walls of the pond, the bacteria loves to feed on this algae, and as it does so it releases the enzyme into the water. Green water is a pain for many reasons. Ultra Violet Clarifier lights will kill single cell phytoplankton algaethat cause green water, and when dead they clump together and can be removed by the filter. However there is sometimes a concern expressed that passing water through the UVC also kills beneficial bacteria.
Activated Carbon in RAS system (Its posibble to use in CENTS Biofilter)
Typically use activated carbon in three different facets of aquaculture: taking impurities out of water as it is brought into the facility; removing halogens such as ozone, chlorine and bromine; and removing color and metabolic by-products in recirculating systems. Activated carbon is the generic term used to describe the family of carbonaceous adsorbents with an extensively developed internal pore structure. A wide variety of activated carbon products are available, exhibiting markedly different characteristics.
They are commonly made from wood, coal, lignite and coconut shell.In activated carbon's manufacture, the material is first subjected to a heating process called carbonization, which forms a fixed carbon mass full of tiny pores. It is then activated by a second heat/steam treatment (200–1,600°C) while regulating oxygen level, which creates an even larger internal pore network and imparts surface chemistries that give carbon its unique filtering characteristics. Some carbons are activated with phosphoric acid, potassium hydroxide or zinc chloride, which makes them unsuitable for use in aquaculture. When selecting an activated carbon, consider the adsorptive characteristics of that carbon on the chemicals to be removed.Activated carbon’s adsorptive characteristics are based on the principle that the greater the surface area, the higher the number of adsorptive sites available.
The pore size and the pore size distribution are extremely important, as they affect the efficacy of the carbon. The macropores (larger than 25 nm) are used as the entrance to the carbon, the mesopores (1–25 nm) for transportation and the micropores (less than 1 nm) for adsorption. It is a generalization to say that the porosity of an activated carbon can be measured by adsorption of iodine from solution, but this measurement may not at all predict its ability to adsorb other chemicals.The finer the particle size of an activated carbon, the better the access to the surface area and the faster the rate of adsorption. Small pore size must be weighed against pressure drop, as this will affect energy cost.
Careful consideration of particle size can provide significant operating benefits.Activated carbon will adsorb the following from water: chlorine and some chloramines, many dissolved organic contaminants, trihalomethanes (THM) and phenolics, total organic carbon (TOC), oil and hydrocarbon contamination, ozone, bromic acid and total organic halogens (TOX), adsorbable organic halogens (AOX) including chloroform, colors, pesticides, odors and more. Activated carbon will also reduce biological oxygen demand (BOD) and chemical oxygen demand (COD).It is important to be able to measure the contaminant that the carbon needs to adsorb in order to know when the saturation capacity of the carbon is reached. '
Particle size, water flow rate, carbon bed depth and, in recirculating systems, the number of passes through the bed must be optimized for every system design. Typically, for a single pass system, a deep bed with very slow flow rates would be required, so that removal of dissolved organics can take place in the top portion of the bed. Change the carbon before it becomes saturated. If the carbon is not replaced, it could desorb what it has already removed. This can cause a nasty, toxic release. Always backwash the filter before use. In backwashing, a bed expansion of at least 25 percent should be used to remove any carbon dust.If it is absolutely necessary to remove a contaminant from the water, use a series of activated carbon filters and do water sampling after the first filter.
The second filter will act as guard bed. Carbon, like all surfaces in recirculating aquaculture, will support bacteria that consume some of the absorbed organics and, if left too long, can slime over the surfaces. Ozone and chloramines oxidize the carbon's surface, and they do not accumulate in the carbon structure.Carbon filters through both its grain size and by its ability to bind up organic and inorganic materials to itself through an electrical charge on its surface. This is known as Adsorption.Carbon filtration is used successfully in industry for filtering wastewater, for the removal of fine insoluables from water and to remove metals and chlorine compounds from domestic water. Carbon filtration is also used to control biological contamination in water.Activated Coal carbon has a different internal structure than coconut carbon thereby allowing for more uptakes of certain contaminants.
What is activated carbon made from?
Activated carbon can be manufactured from any organic material containing carbon. Commercial carbons are made from sawdust, wood, charcoal, peat, lignite, petroleum coke, bituminous coal, and coconut shells.Activated carbon products made from bituminous coal, coconut shell, and wood. Water Filter Corp chooses these raw materials in order to provide good activated carbon to its customers.How is activated carbon produced?The coal is pulverised to a very fine particle, about the size of talcum powder. The powdered coal is mixed with a binder to glue it back together and pressed into briquettes. These in turn are crushed and classified to the size of the desired end product.This process, called reagglomeration, creates an activated carbon that is harder and less dusty than a direct activation process.Reagglomeration also assures that the activation occurs through the granule to the core. Some direct activation processes only activate the exterior of the granule.
The sized material is heated in an oxygen void environment to avoid burning and to remove the volatile components of the coal. The carbon is activated by additional heating in a controlled environment of oxygen and steam. The activation process creates a highly porous graphitic plate structure with tremendous surface area.
How much surface area does activated carbon have?
A single pound of activated carbon has the surface area equal to 125 acres.How much does it weigh?Pure carbon weighs about 130 pounds per cubic foot. It is much denser than activated carbon. During the manufacturing process the structure is opened up, creating porosity (pore volume) inside the granule. The finished product has a density between 25 to 40 pounds per cubic foot.How much void space is in carbon?A container of carbon is roughly 20% carbon, 40% interstitial space (the volume between the carbon granules), and 40% pore volume (the volume inside the carbon granules).Another way to visualise this is: If you had a 55 gallon drum full of dry carbon, you could add 44 gallons of water to the drum before it would overflow. Therefore, 80% of the drum volume is air.What is this pore space?The pore space is the internal volume of the carbon granule. The pore space consists of all the cracks and crevices created when the coal is crushed and glued back together, and the volume between the graphite plates. The distance between the graphite plates determines whether the space is an adsorption pore or a transport pore.What is an adsorption pore?Adsorption pores are the internal volume where the graphitic plates are close together creating a higher energy. Higher energy is important to adsorption because it is the energy that holds the contaminant (the carbon adsorbs the contaminant).The volume where the graphite plates are far apart and the cracks and crevices make up the transport pores. It is important to note that all adsorption takes place in the adsorption pores and not the transport pores.
Zeolite can remove ammonia in RAS

Oxygen and ammonia are the two most important parameters in aquaculture operations. While oxygen can be easily controlled ammonia on the other hand is much more difficult to mitigate and is highly detrimental to the health of fish. The natural generation of toxic levels of ammonia (NH3) and hydrogen sulphide (H2S) by large densities of fish in aquaculture operations affects fish tissue, growth rates, oxygen utilization, disease resistance and causes mass mortality.Zeolite is currently used in commercial fish farms to reduce ammonium (NH4) and hydrogen sulphide levels resulting in increased growth rates and population densities. Zeolite is also used during fish transportation allowing the delivery of more fish over a longer period of time.
Zeolite has a high selectivity and capacity for ammonium via cation exchange capacity (CEC). Once the ammonium ion is within the zeolite lattice, it is not water-soluble. When used as an ion-exchange filter medium the zeolite can reduce ammonium content of circulating freshwater from aquaculture systems by as much as 97%. Piper and Smith (1982) suggested that a water recycling system with a zeolite filter system can allow up to a 10 fold increase in fish density. Zeolite also reduces ammonium content in discharge waters in order to meet environmental requirements.Zeolite is 100% natural, durable and can also be simply regenerated using a brine solution (with a rinse) for repeat cycles of this ion exchange filter bed. Zeolite can also be broadcast over the surface of a pond to be effective in reducing ammonium. The pond-bed sludge can be recovered and used as a nutrient enriched slow release fertilizer.
The required zeolite quantity for your operation depends on water pH, temperature, volume along with fish species and population density. When the optimum quantity of zeolite is used, the ammonium level is reduced at a rate highly dependent upon the rate of water movement. A variety of systems have been designed for reduction of ammonia in fish rearing environments.Zeolite also provides a substrate for bacterial populations in order to breakdown ammonium to nitrate (NH4 to NO3) and remains effective as a chemical filter capable of modifying fluctuations in the system's ammonium levels. This enhances the biological functions making ammonium available to bacteria at a stable level, thus enabling the bacteria to remain abundant during periods of low ammonium contamination. The bacterial population will therefore survive during dramatic changes in concentration.There are three filtration processes to reduce ammonia in the water:Mechanical filtration of unused food and fecal material. Zeolite is much more effective than sand and charcoal filters due to nominal rating of 3 to 5 microns (sand is typically 20 microns) thereby increasing loading while reducing the amount of backwashing. Refer to the ‘Water Filtration’ menu item in the Industrial Section.Biological.
The required zeolite quantity for your operation depends on water pH, temperature, volume along with fish species and population density. When the optimum quantity of zeolite is used, the ammonium level is reduced at a rate highly dependent upon the rate of water movement. A variety of systems have been designed for reduction of ammonia in fish rearing environments.Zeolite also provides a substrate for bacterial populations in order to breakdown ammonium to nitrate (NH4 to NO3) and remains effective as a chemical filter capable of modifying fluctuations in the system's ammonium levels. This enhances the biological functions making ammonium available to bacteria at a stable level, thus enabling the bacteria to remain abundant during periods of low ammonium contamination. The bacterial population will therefore survive during dramatic changes in concentration.There are three filtration processes to reduce ammonia in the water:Mechanical filtration of unused food and fecal material. Zeolite is much more effective than sand and charcoal filters due to nominal rating of 3 to 5 microns (sand is typically 20 microns) thereby increasing loading while reducing the amount of backwashing. Refer to the ‘Water Filtration’ menu item in the Industrial Section.Biological.
The tremendous surface area and irregular surface of BRZ Zeolite provides an idea media for aerobic bacteria. The bacteria mineralize the organic nitrogen compounds. The process can be aerobic (nitrification) or anaerobic (denitrification). Nitrification is most common and involves the oxidation of ammonia to nitrite to nitrate by autotrophic bacteria.Chemical . Due to its large cation exchange capacity zeolite is an excellent filter of ammonium and certain heavy metals.
Isnin, 4 Mei 2009
Wheat Straw can be used as biofilter media / Aquacultural Engineering (2007) 37, 222-233
Soares and Abeliovich (1998) and Aslan and Turkman(2003) indicate that wheat straw can be used as biofilter media and as a carbon source for the denitrification of drinking water. Lowengart et al. (1993) also used wheat straw to denitrify turbid and nitrogen-rich irrigation water. Similarly, Blowes et al. (1994) have demonstrated that wood chips can be used to as a biofilter media to treat runoff and irrigation water. Kim et al.(2003) investigated the use of both wood chips and wheat straw for nitrate removal in a bioretention study. Robertson et al. (2000) have evaluated sawdust, leafcompost, unprocessed grain seeds and wood mulch as reactive barriers to the flow of nitrate-laden waters. More recently, Robertson et al. (2005) reported on a commercially available wood-based biofilter media (marketed as NitrexTM) to remove nitrate–nitrogen froma pretreated residential septic tank effluent. Volokitaet al. (1996) studied shredded newspaper as a biofilter media in denitrification columns.
Study evaluated wood chips and wheat straw as inexpensive and readily available alternatives to more expensive plasticmedia for denitrification processes in treating aquaculture wastewaters or other high nitrate waters. Nine 3.8-L laboratory scale reactors (40 cm packed height  10 cm diameter) were used to compare the performance of wood chips, wheat straw, and Kaldnes plastic media in the removal of nitrate from synthetic aquaculture wastewater. These upflow bioreactors were loaded at a constant flow rate and three influent NO3–N concentrations of 50, 120, and 200 mg/L each for at least 4 weeks, in sequence. These experiments showed that both wood chips and wheat straw produced comparable denitrification rates to the Kaldnes plastic media. As much as 99% of nitrate was removed from the wastewater of 200 mg NO3–N/L influent concentration. Pseudo-steady state denitrification rates for 200 mg NO3–N/L influent concentrations averaged (1360 Æ 40) g N/(m3 d) for wood chips,(1360 Æ 80) g N/(m3 d) for wheat straw, and (1330 Æ 70) g N/(m3 d) for Kaldnes media. These values were not the maximumpotential of the reactors as nitrate profiles up through the reactors indicated that nitrate reductions in the lower half of the reactors were more than double the averages for the whole reactor. COD consumption per unit of NO3–N removed was highest with the Kaldnes media (3.41–3.95) compared to wood chips (3.34–3.64) and wheat straw (3.26–3.46). Effluent ammonia concentrations were near zero while nitrites were around 2.0 mg NO2–N/L for all reactor types and loading rates. During the denitrification process, alkalinity and pH increased while the oxidation–reduction potential decreased with nitrate removal. Wood chips and wheat straw lost 16.2% and 37.7% of their masses, respectively, during the 140-day experiment. There were signs of physical degradation that included discoloration and structural transformation. The carbon to nitrogen ratio of the mediaalso decreased. Both wood chips and wheat straw can be used as filter media for biological denitrification, but time limitations forthe life of both materials must be considered.
Sabtu, 25 April 2009
Langgan:
Catatan (Atom)
Where you can see Cents Flowthrough and CENTS RAS ?
View Marine Finfish Production and Research Center in a larger map